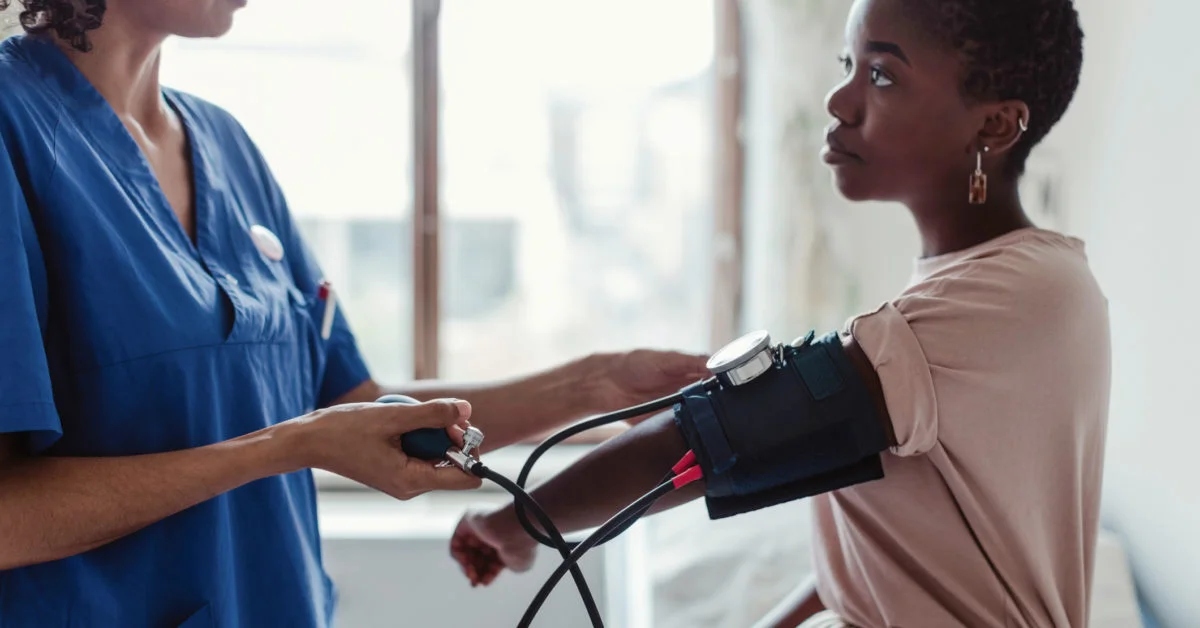
Clinical trials are incredibly important to the advancement of new treatments, but they are also a monocultural space. This is bad news for people who use medications disproportionately, such as women and African Americans. In other words, clinical trials are a bit of a boys club. When you think about it this way, it’s no wonder that the FDA has been forced to follow up on so many safety issues after drugs were approved by regulators. Thankfully, there are some simple steps that can be taken for improving patient diversity in clinical trials:
Use modern recruitment and retention technologies
A great way to make your clinical trial experience as easy and seamless as possible is to use modern recruitment and retention technologies. With a chatbot, for instance, you can answer questions that patients may have about their involvement with the study. A website will help them register for the trial in one place, and a mobile app will make it even more convenient by allowing them to sign-up on their phone instead of having to do it on desktop or laptop computers at home. You could also use text messaging to remind people of upcoming appointments or other important dates (such as when they’ll receive their medication).
Offer direct incentives to patients who participate in clinical trials
These incentives should include incentives for patients who complete the trial, such as a gift card or donation to charity. As per the experts at Medable, “These can be used to encourage diversity in clinical trials by targeting low-income and minority populations that tend to be underrepresented in drug development studies.”
Advertise your clinical trials on the right channels
We do not want to encourage you to advertise your clinical trials on the wrong channels. For example, advertising on platforms that have a low user base of the target population can be an ineffective method of reaching new patients. The same goes for ads that are confusing or difficult to understand or ones that don’t have a clear call to action (CTA).
Offer flexible participation parameters to patients who are excluded from trials
Patients who are too old or too young, patients with certain medical conditions, patients who aren’t able to travel for long periods of time, and those unwilling to be in the study for long periods may not be eligible for certain trials. If you fall into any of these categories, don’t despair: there are still ways you can participate in clinical trials.
Let’s say you’re an elderly patient and have been excluded from a trial due to your age. You still might be eligible for another trial that is looking for older participants—just because one study doesn’t want someone your age doesn’t mean another won’t! Or perhaps it’s not just your age keeping you out; maybe they’ve also excluded people with certain medical conditions.
Create a welcoming and diverse research environment
For this, you have to:
- Provide clear information about clinical trials.
- Provide comfortable waiting areas, refreshments, and snacks.
- Offer child care services (if needed).
- Include diverse representation in the research team.
Establishing a diverse research environment is not easy, but it’s a necessary step toward finding better treatments. The best way to make progress on this is by improving recruitment and retention strategies so that more people can participate in clinical trials.